Osmosis: definition
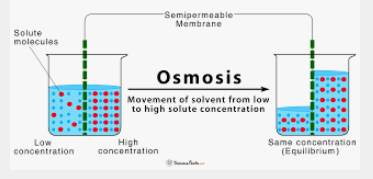
Osmosis is the diffusion of water molecules across a selectively permeable membrane from a region of high concentration to a low concentration. The selectively permeable membrane allows only water molecules to pass through while blocking other substances.
Osmosis is a passive process, meaning it requires no energy input. It occurs due to the concentration gradient or the difference in the concentration of water molecules on either side of the membrane. The concentration gradient drives water molecules to diffuse across the membrane in order to equalize the concentration on both sides.
In osmosis, the water moves from an area of high concentration (known as the hypotonic solution) to an area of low concentration (known as the hypertonic solution). If a cell is placed in a hypotonic solution, it will absorb water and swell, while if it is placed in a hypertonic solution, it will lose water and shrink.
Osmosis is essential for the survival of many organisms, including plants and animals. For example, in plants, osmosis plays a crucial role in transporting water and nutrients from the roots to the rest of the plant. Additionally, in animals, osmosis helps regulate the water balance in the cells and prevents dehydration.
Osmosis: various processes
The process of osmosis can be described by the following steps:
- The concentration of water molecules is higher in the hypotonic solution compared to the hypertonic solution.
- The water molecules move from the hypotonic solution to the hypertonic solution, crossing the selectively permeable membrane.
- The movement of water molecules continues until the concentration of water molecules on both sides of the membrane is equal.
Osmosis: affected by various factors
The rate of osmosis can be affected by several factors, including the size of the concentration gradient, the permeability of the membrane, and the temperature. The larger the concentration gradient, the faster the rate of osmosis will be. On the other hand, the higher the temperature, the faster the rate of molecular movement will be, thus increasing the rate of osmosis.
Osmosis: practical applications
Osmosis is also used in many practical applications, including food processing and purifying water. For example, in the food industry, osmosis is used to preserve fruits and vegetables by removing water from them and replacing it with a sugar solution. This process slows down the growth of bacteria and other microorganisms, which would otherwise cause the food to spoil.
Osmosis: other related information
Osmosis is a necessary process that occurs in both living and non-living systems. It is a passive process that allows water molecules to diffuse from an area of high concentration to an area of low concentration, driven by the concentration gradient. Osmosis is crucial for many organisms’ survival and is also used in several practical applications, including food processing and purifying water.
Osmosis can also be observed in reverse, where water moves from a region of low concentration to a region of high concentration. This is called reverse osmosis, and it is a technique used to purify water by removing salts, minerals, and other impurities. Reverse osmosis applies pressure to the hypertonic side of the selectively permeable membrane, forcing water to flow in the opposite direction of the concentration gradient. This process is commonly used in desalination plants to produce fresh water from seawater.
In addition to reverse osmosis, osmosis can also be used in a process called dialysis. Dialysis is a medical treatment that removes excess fluid, waste products, and toxins from the bloodstream. In dialysis, a patient’s blood is pumped through a semi-permeable membrane, which acts like a selectively permeable membrane, allowing only certain substances to pass through. This process is used to treat conditions such as kidney failure, where the kidneys can no longer function effectively and remove waste products from the body.
Osmosis is also a crucial process in the transport of water in plants. In plants, water enters the roots and moves up the stem to the leaves through a process called transpiration. During transpiration, water is absorbed by the roots and then moves up the stem, where it is lost to the atmosphere through tiny pores in the leaves called stomata. This water loss creates a negative pressure in the stem, drawing more water from the roots in a continuous cycle. This process helps to distribute water and nutrients throughout the plant and maintain turgor pressure, which is the pressure that prevents wilting.
In summary, osmosis is a fascinating and widely applicable process that plays a crucial role in many biological and technological systems. From maintaining cell hydration to purifying water and removing waste from the bloodstream, osmosis is a fundamental process that is essential for life and plays a key role in many vital processes.
Here are some examination questions on osmosis
- What is osmosis, and how does it work?
- What is the concentration gradient, and why is it important for osmosis?
- What is the difference between a hypotonic solution and a hypertonic solution?
- What are the factors that affect the rate of osmosis?
- What is reverse osmosis, and how is it used?
- What is dialysis, and how does it use the principles of osmosis?
- Explain the role of osmosis in the transport of water and nutrients in plants.
- Describe an experiment to demonstrate the process of osmosis.
- What is turgor pressure, and how is it maintained in plants through osmosis?
- Discuss the practical applications of osmosis in food and water purification.
These titles aim to highlight the various aspects of osmosis and its impact on biological, agricultural, medical, and technological systems. You can use these titles as inspiration to craft a title that best fits your specific blog post and target audience.